Electrical Conductivity Of Salt Water Solutions
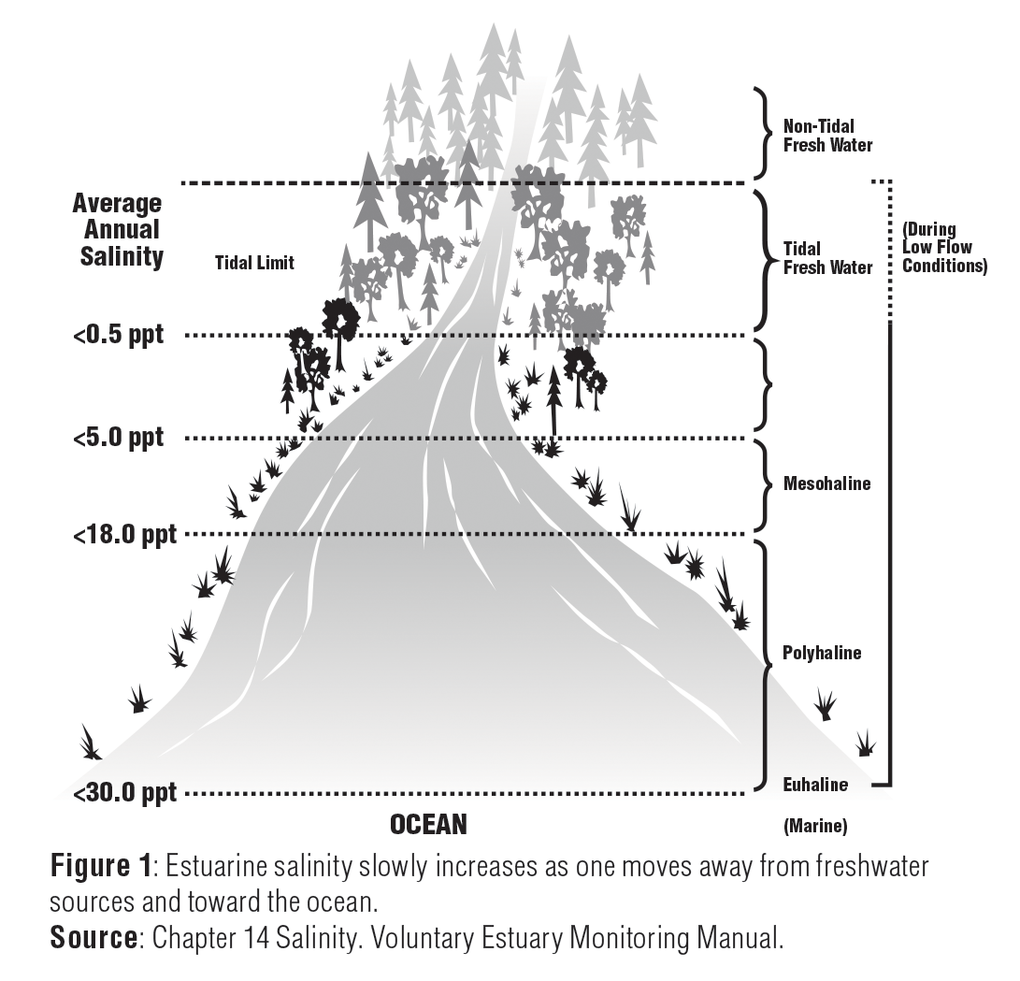
The purer the water the lower the conductivity the higher the resistivity.
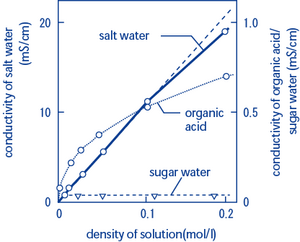
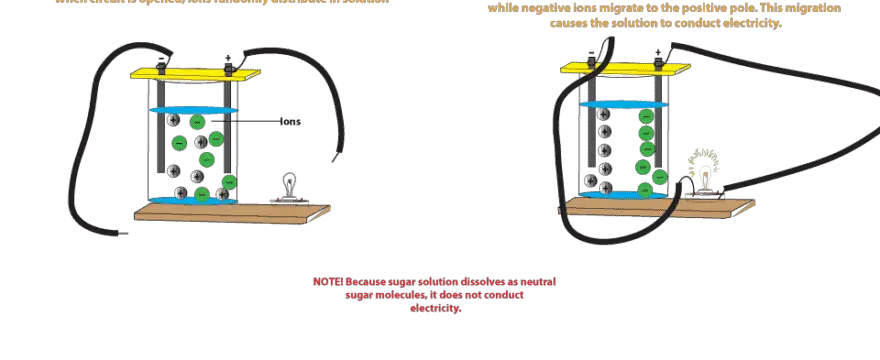
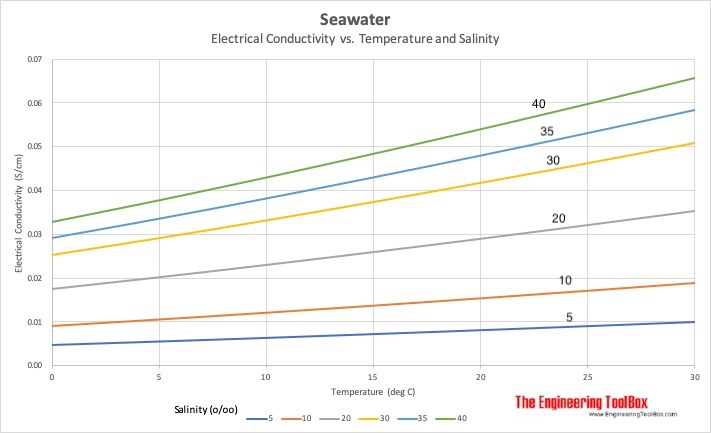
Electrical conductivity of salt water solutions. As the current flows the ions move to the electrodes placed in the solution. Conductivity is a measure of water s capability to pass electrical flow. Ions must be present in solution for electrical conductivity. Salts dissolve in water to produce an anion and a cation.
In many cases conductivity is linked directly to the total dissolved solids t d s. Conductivity is traditionally determined by connecting the electrolyte in a wheatstone bridge. An ion is an atom that has an electrical charge because it has either gained or lost an electron also meaning it has a positive charge and a negative charge. These ions make up the basis of conductivity in water.
Salt acid and alkali solutions containing ions are called electrolytes. The conductivity of a solution of water is highly dependent on its concentration of dissolved salts and other chemical species that ionize in the solution. The ions carry the electric charge through the solution thus creating an electric current. Conductivity is the ability of water to conduct an electrical current and the dissolved ions are the conductors.
To test the conductivity of salt water vs fresh water we can perform a simple and fun experiment. This ability is directly related to the concentration of ions in the water 1. Electrical conductivity of water samples is used as an indicator of how salt free ion free or impurity free the sample is. If a light bulb in the circuit lights up a current is flowing in the circuit.
Strong acids and salts are strong electrolytes because they completely ionize dissociate or separate in solution. The major positively charged ions are sodium na calcium ca 2 potassium k and magnesium mg 2. Salt molecules are made of sodium ions and chlorine ions. This is because salt water is a good conductor of electricity which makes ocean water a resource for renewable energy.
Salts that dissolve in water break into positively and negatively charged ions. The current if sufficient enough will light one or both leds on a conductivity meter shown at right.












.png)