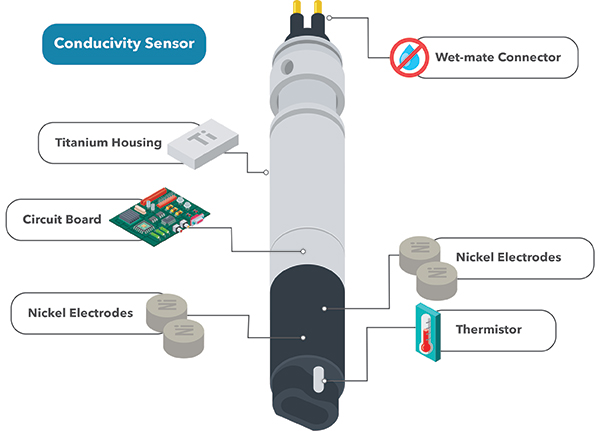
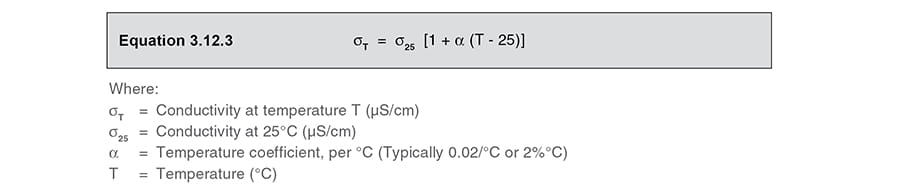
Electrical Conductivity Of Water Formula

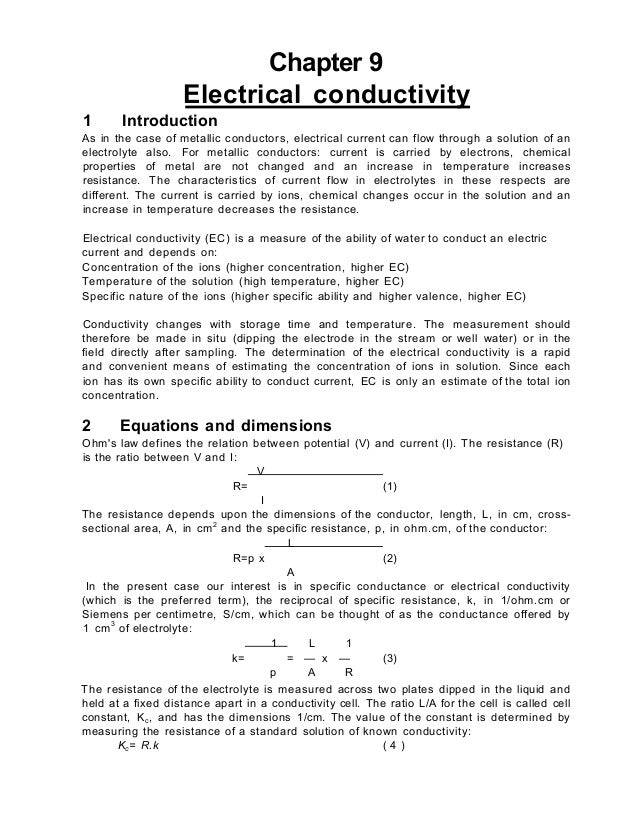
The purer the water the lower the conductivity the higher the resistivity.
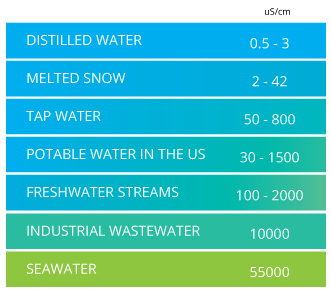
Electrical conductivity of water formula. These conductive ions are originated due to inorganic materials such as chlorides alkalis carbonate and sulfides compounds and dissolved salts. Using this diagram it is possible to relate the conductivity to the resistance length and cross sectional area of the specimen in the conductivity formula below. σ r l a r σ a l. Electrical conductivity of water samples is used as an indicator of how salt free ion free or impurity free the sample is.
The conductivity is from the resistivity conductivity relation. These conductive ions come from dissolved salts and inorganic materials such as alkalis chlorides sulfides and carbonate compounds 3. From the electric resistance formula we find. The electrical conductivity of water increases by 2 3 for an increase of 1 degree celsius of water temperature.
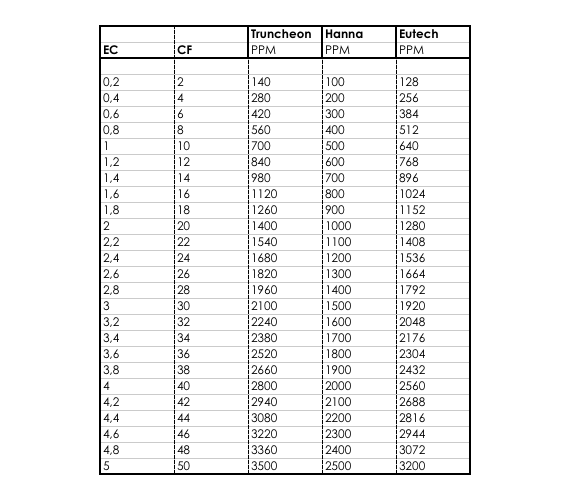
The major positively charged ions are sodium na calcium ca 2 potassium k and magnesium mg 2. Conductivity is the ability of water to conduct an electrical current and the dissolved ions are the conductors. Conductivity is a measure of water s capability to pass electrical flow. Ec 0 055 µs cm at 25 c.
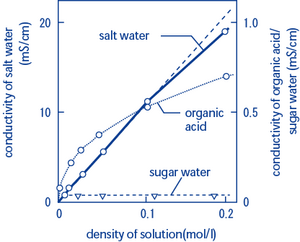
ρ r a l 1 53 k ω 0 0003 m 3 1 m 4 6 10 1 ω m. Electrical conductivity is defined as the ratio between the current density j and the electric field intensity e and it is the opposite of the resistivity r w m. This ability is directly dependent on the concentration of conductive ions present in the water. While the electrical conductivity is a good indicator of the total salinity it still does not provide any information about the ion composition in the water.
In water and ionic materials or fluids a net motion of charged ions can occur. σ 1 ρ 2 1 s m. Many ec meters nowadays automatically standardize the readings to 25 o c. This phenomenon produce an electric current and is called ionic conduction.
S j e 1 r. This ability is directly related to the concentration of ions in the water 1. Due to the self ionization of water into h und oh ions the electrical conductivity of pure water is non zero.